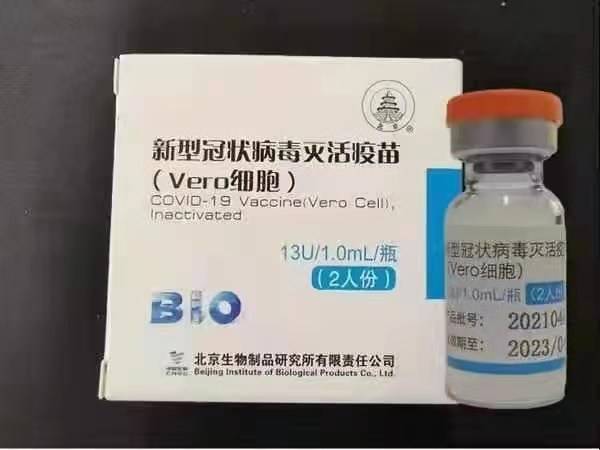
ISinopharm (eBeijing): BBIBP-CorV
ISIGABA 1
1 Uvavanyo
I-ChiCTR2000032459
China
ISIGABA 2
Izilingo ezi-2
I-NCT04962906
Ajentina
I-ChiCTR2000032459
China
ISIGABA 3
Izilingo
I-NCT04984408
I-ChiCTR2000034780
IUnited Arab Emirates
I-NCT04612972
Peru
I-NCT04510207
IBahrain, i-Egypt, iJordani, i-United Arab Emirates
I-NCT04560881, IBPP2020003AR
Ajentina
I-NCT04917523
IUnited Arab Emirates
Ukuvunywa
Uluhlu lokuSebenzisa ngokungxamisekileyo lwe-WHO Amazwe angama-59
Angola 、 Argentina 、 Bahrain 、 Bangladesh 、 Belarus 、 Belize 、 Bolivia (State Plurinational of) 、 Brazil Brunei Darussalam 、 Cambodia 、 Cameroon 、 Chad 、 China 、 Comoros 、 Egypt 、 Equatorial Guinea 、 Gabon ambia Gambia 、 Georgia 、 Guyana 、 Hungary 、 ndonesia 、Iran (iRiphabliki yamaSilamsi) 、 iIraq 、 iJordani 、 eKyrgyzstan 、 kwiRiphabhlikhi yaseLao Yabantu
ILebhanon eMalaysia iMaldives 、 iMauritania 、 iuritus 、 iMongolia 、 iMontenegro 、 iMorocco iMozambiki 、 Namibia 、 Nepal 、 Niger 、 North Macedonia 、 Pakistan 、 Paraguay 、 Peru 、 Philippines 、 Republic of the Congo 、 enegal 、 Serbia 、 Seychelles 、 Sierra Leone 、 Solomon Iziqithi 、 ISomalia 、 iSri Lanka 、 iThailand 、 iTrinidad neTobago 、 iTunisia 、 I-United Arab Emirates 、 iVenezuela (kwiRiphabliki yaseBolivaria) 、 Viet Nam 、 Zimbabwe
ISinopharm BBIBP-CorV COVID-19 sisitofu esingasebenziyo esenziwe ngamasuntswana egciwane elikhuliswe ngokwenkcubeko elingenakho ukubangela izifo. Lo mgqatswa wokugonya waphuhliswa yiSinopharm Holdings kunye neBeijing Institute of Biological Products.
Isitofu sokugonya seSinopharm BBIBP-CorV sisebenza ngokuvumela amajoni omzimba ukuba avelise izilwa-buhlungu ezichasene ne-SARS-CoV-2 beta coronavirus. Izitofu zokugonya ezingasebenziyo ziye zasetyenziswa amashumi eminyaka, njengokugonya i-rabies kunye ne-hepatitis A vaccine. Obu buchwephesha bophuhliso busetyenziswe ngempumelelo kwizitofu zokugonya ezininzi ezaziwayo, ezinjengeyeza lokugonya umgada.
Uxinzelelo lweSinopharm's SARS-CoV-2 (ubunzima be-WIV04 kunye nenombolo yethala leencwadi iMN996528) yahlukaniswa nesigulana kwisibhedlele iJinyintan eWuhan, China. Intsholongwane yasasazwa kwinkcubeko kwilayini yeVero efanelekileyo, kwaye iiseli ezingaphezulu kweentsholongwane zazingasebenzi kunye ne-β-propiolactone (1: 4000 vol / vol, 2 ukuya ku-8 ° C) iiyure ezingama-48. Emva kokucaciswa kwemfucumfucu yeseli kunye ne-ultrafiltration, yesibini β-propiolactone inactivation yenziwa phantsi kweemeko ezifanayo nokusetyenziswa kokuqala. Ngokwe-WHO, iyeza lokugonya labhengezwa kwi-0.5 mg ye-alum kwaye yafakwa kwisirinji ekhethiweyo kwi-0.5 mL ye-saline phosphate-buffered saline ngaphandle kokubulala.
Nge-31 kaDisemba ngo-2020, uLawulo lwezoNyango lukaRhulumente lwabhengeza ukuvunywa kwesitofu sokulinga esenziwe nguSinopharm.
Ngomhla we-7 kaMeyi, 2021, uMbutho wezeMpilo weHlabathi wabhengeza ukuvunywa kwesitofu sokugonya. Uluhlu lokusetyenziswa okungxamisekileyo kwe-WHO lwenze amazwe ukuba akhawulezise ukuvunywa kwawo kolawulo lokungenisa elizweni kunye nokulawula isitofu sokugonya se-COVID-19. Iqela eliCebisayo le-WHO kwiQhinga lokuGonya nalo likugqibile ukuphononongwa kwakhona kwesitofu sokugonya. Ngokusekwe kubo bonke ubungqina obukhoyo, i-WHO icebisa iidosi ezimbini zokugonya, iiveki ezintathu ukuya kwezine ukwahlukana, kubantu abadala abaneminyaka eli-18 nangaphezulu. Ukusebenza kokugonya ngokuchasene nesifo esineempawu kunye nezibhedlele kuqikelelwa kuma-79% kuwo onke amaqela eminyaka edibeneyo.
Umbutho waseMelika woNyango upapashe "Uvavanyo lweKlinikhi olungalungiswanga: Iziphumo zezigonyo ezi-2 ezingasebenziyo ze-SARS-CoV-2 kwi-Symptomatic COVID-19 Yosulelo kubantu abadala" ngoMeyi 26, 2021, bequkumbela ngelithi "kolu hlalutyo luchaziweyo lolingo olungenamkhethe, Abantu abadala Izitofu ezi-2 ezingasebenziyo ze-SARS-CoV-2 ezenziwa kolu hlalutyo lwexeshana olucacisiweyo lwezilingo zeklinikhi ezingahleliwe zinciphise kakhulu umngcipheko weempawu ze-COVID-19, kwaye iziganeko ezibi kakhulu bezinqabile. " Kweli nqanaba 3 kuvavanyo olungenamkhethe kubantu abadala, ukusebenza kwe-2 engasebenziyo iyeza lokugonya intsholongwane kwiimeko ze-COVID-19 ezinophawu yayiyi-72.8% kunye ne-78.1%, ngokwahlukeneyo. Izitofu ezi-2 zazineziganeko ezimbi ezinqabileyo ezinamaxesha afanayo kwiqela lolawulo lwe-alum kuphela, kwaye uninzi lwalungahambelani nogonyo. Uhlalutyo lokuhlola lufumanise ukuba izitofu ezi-2 zibangele ii-antibodies ezinokulinganiswa ezingalinganiyo, ezifanayo kwiziphumo zesilingo se-1/2.
Iqela elisebenzayo le-WHO SAGE lipapashe uphononongo lwesitofu se-Sinopharm / BBIBP COVID-19 ngoMeyi 10, 2021. Isitofu sokugonya se-GAV sika-COVID-19 siquka i-vial monitor ye-vaccine exelela abasebenzi bezempilo ukuba ingaba isitofu sigcinwe ngokufanelekileyo na kwaye asichanekanga ubushushu. Ngenxa yoko, umonakalo, i-GAVI yanika ingxelo ngoMeyi 14, 2021. iilebhile ezikrelekrele ezenziwe yiZebra Technologies ezenziwe yiTemptime Corporation, zinesangqa esinesikwere esikhanyayo embala embindini, esenziwe ngemichiza engenambala engenakuphinda ivelise umbala ngokuhamba kwexesha. . Oku kuba mnyama ukunika umbono obonakalayo wokunyuka kobushushu. Nje ukuba ibhotile ivezwe kubushushu obungaphaya kobude bayo obugciniweyo, isikwere siba mnyama kunesangqa, sibonisa ukuba isitofu sokugonya akufuneki sisetyenziswe.
Inombolo yobhaliso lwamachiza e-National Drug BBIBP-CorV COVID-19 yamayeza okugonya: DB15807.